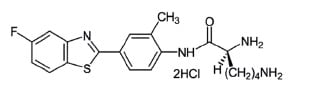
Antitumoral Phortress Small Molecule (Tool Compound)
Invented at University of Nottingham
- Datasheet
- References (6)
- Inventor Info
Info
| Catalogue Number | 151839 |
| Antigen/Gene or Protein Targets | Human derived carcinomas of breast, ovarian and renal origin |
| Type | Antitumoral |
| Relevance |
The Antitumoral Phortress compound is a potential anticancer agent for treatment of human breast carcinoma. Phortress is highly selective for susceptible cancer cells because of its mechanism of action. Following the release of 5F 203 from Phortress, it activates AhR signalling and causes induction of cytochrome P450 activity, which metabolically bioactivates 5F 203 to a cytotoxic species at the tumour site. Phortress (the dihydrochloride salt of the lysylamide prodrug of 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole (5F 203)) is an experimental antitumour agent with potent and selective activity against human-derived carcinomas of breast, ovarian and renal origin The mechanism of action of Phortress is distinct from all classes of chemotherapeutic agents currently in the clinic, and involves metabolic activation by cytochrome P450 (CYP) 1A1 to electrophilic species, which generate DNA adducts in sensitive tumours only. |
| On Target IC50 | IC50 values for MCF-7 and MDA468 cells are 40 nM and 158 nM, respectively |
| Molecular Formula | C20H25Cl2FN4OS |
| Molecular Weight (g/mol) | 459.41 |
| In vivo applications | Active against breast, ovarian and renal carcinomas. Taken up into sensitive cells followed by aryl hydrocarbon receptor binding and translocation into the nucleus. Requires metabolic activation by cytochrome P450 to generate cytotoxic species. |
| In vitro applications | Induces expression of CYP1A1 and generates adducts in the DNA of sensitive MCF7 and IGROV-1 cells. |
| Research Area | Cancer |
| Notes |
Compound available for screening or similar studies. For larger quantities (>20mg) please contact Ximbio. Product Documents: Phortress Diode Array Phortress NMR Phortress LCMS Phortress Comparison |
References: 6 entries
Bradshaw et al. 2009. Pharmacology. 83(2):99-109. PMID: 19088497.
Preclinical toxicokinetic evaluation of phortress [2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole lysylamide dihydrochloride] in two rodent species.
Europe PMC ID: 19088497
Fichtner et al. 2004. Breast Cancer Res Treat. 87(1):97-107. PMID: 15377855.
The experimental antitumor agents Phortress and doxorubicin are equiactive against human-derived breast carcinoma xenograft models.
Europe PMC ID: 15377855
Bradshaw et al. 2004. Curr Med Chem. 11(8):1009-21. PMID: 15078163.
The development of the antitumour benzothiazole prodrug, Phortress, as a clinical candidate.
Europe PMC ID: 15078163
Add a reference
References: 6 entries
Bradshaw et al. 2009. Pharmacology. 83(2):99-109. PMID: 19088497.
Preclinical toxicokinetic evaluation of phortress [2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole lysylamide dihydrochloride] in two rodent species.
Fichtner et al. 2004. Breast Cancer Res Treat. 87(1):97-107. PMID: 15377855.
The experimental antitumor agents Phortress and doxorubicin are equiactive against human-derived breast carcinoma xenograft models.
Bradshaw et al. 2004. Curr Med Chem. 11(8):1009-21. PMID: 15078163.
The development of the antitumour benzothiazole prodrug, Phortress, as a clinical candidate.
Add a reference