Anti-RA015/11.88 [11.88] recombinant antibody
Invented at Queen Mary University of London
- Datasheet
- References (1)
- Inventor Info
Info
| Catalogue Number | 160520 |
| Applications | ELISA WB |
| Antigen/Gene or Protein Targets | Neutrophil Extracellular Trap Antigen |
| Synonyms | NET (Neutrophil Extracellular Trap) |
| Relevance |
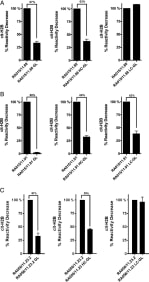
Rheumatoid arthritis (RA) is a joint-destructive inflammatory disorder characterized by breach of self-tolerance and production of anti–cit-peptide/protein Abs (ACPA). In the RA synovium, ectopic germinal centers (GCs) support an autoantigen-driven immune response leading to local ACPA+ B cell differentiation (1, 2). Recently, we reported that autoreactive B cells highly mutated within ectopic GCs frequently target cit-histones (cit-H2A/B) contained in neutrophil extracellular traps (NETs) (3). Somatic hypermutation (SHM) within GCs introduces single-point mutations in the variable heavy (VH) and/or variable light (VL) region of unmutated (germline) BCR, thus regulating Ag-driven B cell affinity maturation (4). Additionally, SHM can introduce N-glycosylation sites in the VH/VL regions, which can influence Ag binding and/or give an advantage during the selection process to autoreactive B cells (5–7). Circulating and synovial fluid ACPA-IgG are extensively N-glycosylated in their Fab domain and this is due to introduction of N-glycosylation sites during SHM. The biological effects mediated by the glycans in the variable domain of ACPA-IgG might modulate either the Ag binding and/or BCR signalling or might influence the binding to lectins thus giving survival signals to autoreactive B cells (5, 7, 8). Therefore, additional studies are necessary to enhance our understanding of ACPA-IgG Fab N-glycans. In particular, a direct demonstration of the relative contribution of SHM in the VH versus VL region and of the importance of Fab N-glycosylation sites for synovial B cell recognition of cit-Ags is missing. Therefore, in this study we characterized the requirement for SHM within the VH and VL regions and of Fab N-linked glycosylation for the immunoreactivity to NETs and cit-H2B in RA-rmAbs derived from CD19+ B cells obtained from ectopic lymphoid structure (ELS)+ RA synovial tissues. In particular, we present three different scenarios whereby 1) SHM in the VH region is sufficient for the binding to NETs/cit-H2B; 2) both VH and VL chain affinity maturation contribute to the immunoreactivity; and 3) the introduction of a single Fab N-glycosylation site account for most of the RA-rmAbs binding to cit-H2B. PMID: 32221039 J Immunol. 2020 May 1;204(9):2374-2379. doi: 10.4049/jimmunol.1901457. Epub 2020 Mar 27. |
| Host | Human |
| Research Area | Neurobiology, Drug Discovery & Development |
| Immunogen UniProt ID | TBD |
| Notes |
Recombinant antibody derived from CD19+ B cells within Rheumatoid Arthritis human synovial tissues. cDNA from single B cells was amplified by nested PCR using IgV gene-specific primers. Amplified Ig VH and VL genes were then cloned and expressed as recombinant antibodies displaying identical specificity of the original B cells. |


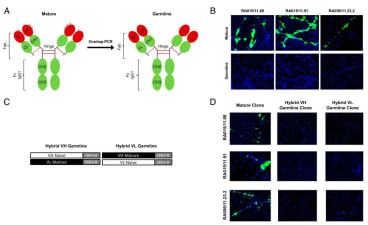
![Image thumbnail for Anti-RA015/11.88 [11.88] recombinant antibody](https://res.cloudinary.com/ximbio/image/upload/c_fit,fl_lossy,q_auto/69a24845-8937-4d89-9603-925c153f80e1.jpg)
![Image thumbnail for Anti-RA015/11.88 [11.88] recombinant antibody](https://res.cloudinary.com/ximbio/image/upload/c_fit,fl_lossy,q_auto/c4461a9e-4ce9-43a6-abf1-8c2888b98ea8.jpg)
![Image thumbnail for Anti-RA015/11.88 [11.88] recombinant antibody](https://res.cloudinary.com/ximbio/image/upload/c_fit,fl_lossy,h_45,q_auto/69a24845-8937-4d89-9603-925c153f80e1.jpg)
![Image thumbnail for Anti-RA015/11.88 [11.88] recombinant antibody](https://res.cloudinary.com/ximbio/image/upload/c_fit,fl_lossy,h_45,q_auto/c4461a9e-4ce9-43a6-abf1-8c2888b98ea8.jpg)
